Project
A combined engineering and molecular approach to study the initiation and progression of tendinopathy
| Primary Investigator: | Hazel Screen |
| Funder: | Arthritis Research UK Funded: MP 18424 |
Tendinopathies are painful and debilitating conditions. Despite their growing prevalence, their aetiology remains poorly understood, with considerable debate as to the roles of mechanical and cellular changes during early disease. This project was focused on developing model systems in order to investigate the early cellular changes in tendinopathy, with a specific interest in inflammatory changes. The role of inflammation in tendinopathy remains unclear, but our data indicates that early inflammatory changes may be an important aspect of the development of tendinopathy.
We developed a system to artificially generate fatigue damage in viable tendon fascicles, in order to investigate their mechanical and metabolic response to different loading conditions. Using this system we have shown that fascicles can withstand low level fatigue loading without resulting sustaining a reduction in mechanical properties. However, even low level fatigue initiates an upregulation of Collagen-1-alpha-chain-1 (COL1A1) and interleukin-6 (IL6) expression, suggesting a role of IL6 in tendon adaptation to exercise.
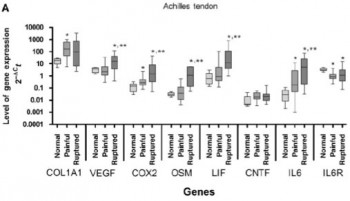
However, we have also shown that COL1A1 and IL-6 expression are increased in tendinopathic tendons, suggesting a role on pathological and well as physiological tendon behaviour. More specifically, we compared gene expression in ruptured and tendinopathic tendons, reporting different gene expression profiles with these conditions, indicating a substantial difference between those two tendinopathies. Inflammatory markers are up-regulated in painful and particularly in ruptured AT, pointing towards a role of inflammation not only in rupture healing, but also in Achilles tendinopathy.
In developing our fatigue model, we have acquired significant data relating to the stress relaxation behaviour of tendon fascicles, and have specifically established the importance of the proteoglycan content of tendon in managing the viscoelastic behaviour, showing significant changes in stress relaxation behaviour in fascicles where GAG has been removed.
Publications Arising:
- Legerlotz K, Riley G.P, Screen H.R.C. (2013) GAG depletion increases the stress relaxation response of tendon fascicles but does not influence recovery. Acta Biomat. 9;6: 6860-6966.
- Legerlotz K, Jones G.C., Screen H.R.C., Riley G.P. (2013) Cyclic loading of tendon fascicles using a novel fatigue loading system increases interleukin-6 expression by tenocytes. Scand J Med Sci Sport. 23:1; 31-37.
- Legerlotz K, Jones E., Screen H.R.C. Riley G.P. (2012) Increased expression of IL6 family members in tendon pathology. Rheumatol. 51:7; 1161-1165
- Legerlotz K, Riley G.P., Screen, H.R.C. (2010) Specimen dimensions influence the apparent material properties of tendon fascicles. J. Biomech. 43:12; 2274-2280.